Plasticity of cell cycle control during development
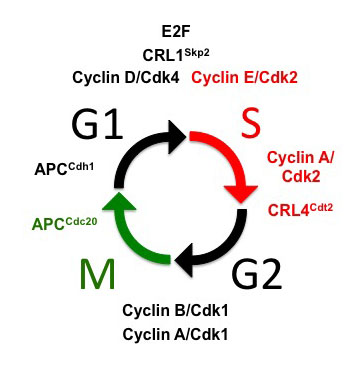
Control of the cell cycle is fundamental to eukaryotic cell biology and is essential for the development and maintenance of every tissue in multi-cellular organisms. Over the last 25 years a powerful molecular paradigm for control of the canonical G1-S-G2-M cell cycle has emerged from studies in both vertebrate and invertebrate model systems. In this view, an oscillating molecular “engine” composed of a family of cyclin dependent kinases (CDKs) ensures the ordered progression of events from one phase to the next, and “checkpoint controls” ensure that one phase is completed before the next is initiated.
However, the paradigm of a highly regulated, 4-phase cell cycle oscillator cannot alone explain the type of regulation that directs the complex patterns of growth necessary for animal development and homeostasis. During animal development the canonical order of cell cycle phases is frequently altered.

A good example occurs during early embryogenesis of many species when the cell cycle lacks gap phases altogether, and constitutive activity of CDKs (e.g. Cyclin E/CDK2) replaces the periodic oscillations that characterize the canonical cell cycle. Another example is the endocycle, in which periods of S phase are interrupted by a gap phase without an intervening mitosis resulting in polyploidy. While there are examples of naturally occurring polyploid cell types in many species, it has also recently become clear that polyploidy can be a source of genomic instability leading to cancer. Therefore, cell cycle plasticity is an inherent feature of development, and its mis-regulation can contribute to disease. One of the important challenges of current cell cycle research and a goal of our research program is to understand the molecular basis for cell cycle plasticity during development.